Objetctive
To compare the live morphology imaging technology of LensHooke® X1 PRO Semen Quality Analyzer with Diff-Quik staining in sperm head morphometric analysis.
Introduction
The dimensions of spermatozoa, especially head parameters, play a pivotal role for the sperm morphology assessment according to WHO 5th manual for human sperm morphology assessment.
Diff-Quik is one of the staining methods recommended by the WHO manual. However, previous studies indicated that the hypotonic characteristics of Diff-Quik fixative and stain solutions might cause the spermatozoa to swell. Therefore, we compared the live morphology imaging technology of LensHooke® X1 Pro with the traditional Diff-Quik staining and microscopic live imaging in osmolality-induced morphological changes (OIMC) on spermatozoa.
Study design
After washed, sperms were resuspended either with or without 200 ppm HgCl2 in ddH2O, human tubal fluid (HTF) and 20% glucose respectively to generate hypo-, iso- and hyper-osmotic settng. Sperm head images were captured via a microscope (1000X, Olympus; BF for Diff-Quik; Ph3 annulus for live imaging) and the LensHooke® X1 PRO. The dimensions of head parameters i.e. length, width and area were easured either by using the ImageJ software manually or by X1 PRO automatically. A total of 2000 individual sperms from 5 repeated tests were analyzed in each group. All analyses were conducted in a blinded fashion. Statistical analyses included t-test and two-way ANOVA with significance at p < 0.01.
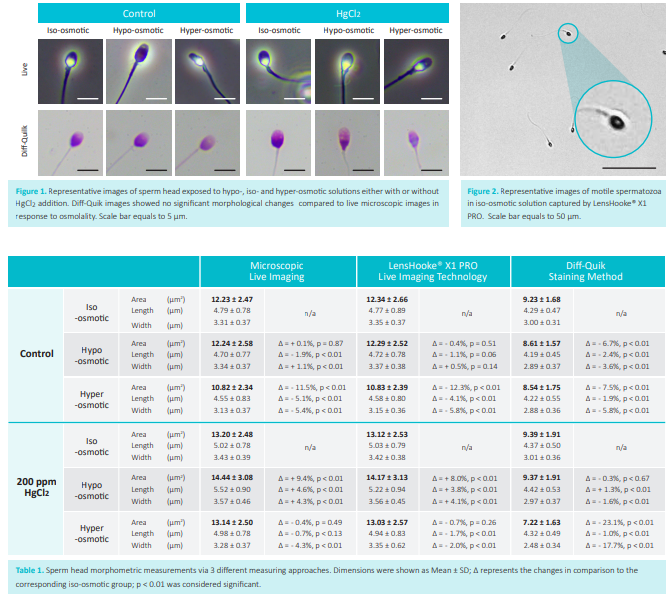
Results
Microscopic live imaging and X1 PRO showed minor changes in head area after ddH2O treatment (0.1%, p = 0.87; – 0.4%, p = 0.51). Hyper-osmotic exposure showed significant shrinkage (-11.5%, p < 0.01; -12.3%, p < 0.01) (Table 1). Head area were dramatically enlarged upon HgCl2 plus hypo-osmotic exposure in both microscopic live imaging and X1 PRO (9.4%, p < 0.01; 8.0%, p < 0.01) (Table 1). HgCl2 plus hyper-osmotic exposure showed no significant difference in both microscopic live imaging and X1 PRO (-0.4%, p = 0.49; -0.7%, p = 0.26) (Table 1). Diff-Quik method displayed inconsistent results of head area either with (hypo: -0.3%, p = 0.67, hyper: -23.1%, p < 0.01) and without HgCl2 addition (hypo: -6.7%, p < 0.01, hyper: -7.5%, p < 0.01) compared with the other methods (Table 1, Fig. 3). X1 PRO took less analyzing time compared to microscopic live imaging (5.1 ± 0.3 vs 319.3 ± 27.4 min, n = 30, p < 0.01 ), there was no significant difference between head morphometrics (p = 0.40) in contrast to Diff-Quik method (p < 0.01).
CONCLUSIONS
LensHooke® X1 Pro live morphology imaging technology is fast and applicable in assessing OIMC. Diff-Quik staining method is not recommended for assessing sperm morphology when OIMC occurred.

