Introduction
Infertility is prevalent in approximately 190 million people and male factor is one of the important reason (50% of the total cases) couple infertility. Semen analysis is considered as the cornerstone for laboratory evaluation of male infertility. Manual semen analysis can assess the macroscopic (pH, volume and appearance) and microscopic (sperm concentration, % total motility, normal sperm morphology, vitality) parameters of the semen sample.
The samples are classified either as normal or abnormal based on the WHO guidelines (5th edition, 2010). The evaluation of certain microscopic parameters, such as sperm concentration, motility, morphology, is time-consuming and requires extensive training of the operator. Due to high subjectivity it makes challenging to rely on the results of manual semen analysis to evaluate male infertility. Furthermore,
manual semen analysis is also prone to inter-observer variations. To overcome these shortcomings, computer-assisted semen analyzers (CASA) were introduced to replace manual semen analysis.
A modern mini device with inbuilt artificial intelligence optical microscopic (AIOM) technology developed by Bonraybio (Taichung City, Taiwan) allows simple, quick and reliable analysis of semen specimen
from patients with male infertility. The device, LensHooke™ X1 PRO semen quality analyzer is built for in vitro diagnostic use and analyses sperm concentration, total motility, progressive motility, non-progressive motility and sperm morphology. The main objective of the current study was to compare two automated semen quality analysis systems [LensHookeTM X1 PRO (X1 PRO) and IVOS CASA] for accuracy, precision and agreement with laboratory-based manual semen analysis (MSA).
Material and Methods
Semen analysis was conducted using X1 PRO (Bonraybio), IVOS CASA (Hamilton Throne) and MSA according to WHO 5th Edition (2010) guidelines in andrology laboratory. Semen samples (n=31) were obtained from healthy male volunteers with normal semen parameters and infertile men with a minimum
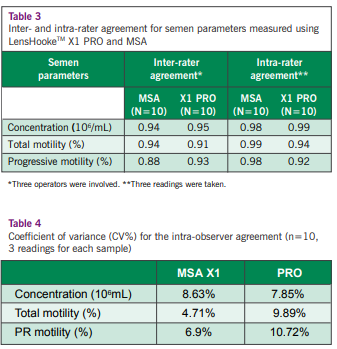
abstinence period of 2 to 3 days. After complete liquefaction, a total of 101 seminal aliquots were prepared and tested using X1 PRO (Figure 1), IVOS CASA and MSA. The test results obtained by X1 PRO and IVOS CASA were compared to MSA using Passing-Bablok regression analysis. Additionally, 10 samples were used to evaluate the intra- and inter-rater agreement for X1 PRO and MSA.
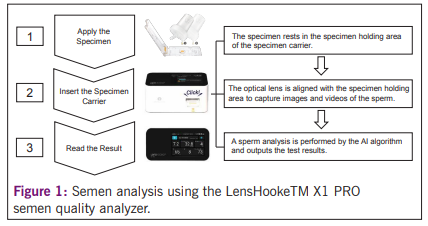
Results
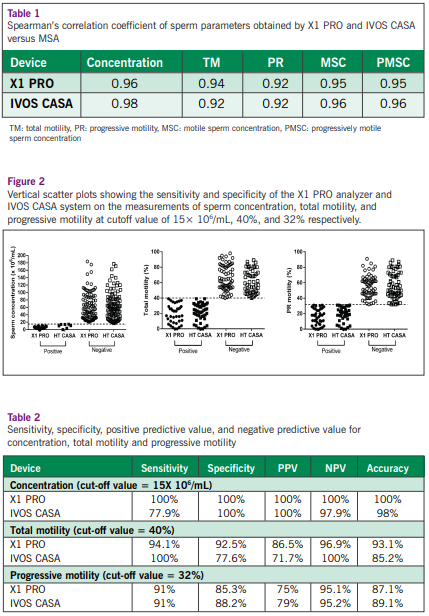
Results
The semen analysis results (sperm concentration, total motility, progressive (PR) motility, motile sperm concentration, and progressively motile sperm concentration) showed strong correlation and agreement for both automated semen analyzers (X1 PRO and IVOS CASA) and MSA with Pearson correlation above 0.92 (P<0.0001) (Table 1). X1 PRO and IVOS CASA were able to differentiate oligozoospermic samples with an accuracy of 100% and 93%, respectively. Furthermore, the positive predictive value for X1 PRO (86.5%) was higher than IVOS CASA (71.7%) in differentiating asthenozoospermic samples (Figure 2 & Table 2). Semen parameter (sperm concentration, total motility and PR motility) showed a high degree of inter- and intra-rater agreement evaluated using X1 PRO and MSA (Table 3). X1 PRO showed an intra-rater precision of coefficient variation, CV <15% for sperm concentration, total motility and PR motility, and was comparable with MSA (Table 4).
Conclusion
Both automated semen analyzers (X1 PRO and IVOS CASA) showed high level of accuracy and precision, and their performance was comparable with laboratory-based MSA

